That is what we've dubbed it "the new alternative, alternative fuel."
A thumbnail view:
- A chemical-catalytic conversion process
- consumes a seemingly endless feedstock supply
- operates without pressure
- converts at the lowest exchange temperature on the market
- has the most favorable net energy balance (consumes only 10% of the energy output)
- produces diesel at a lower cost
- short throughput time of 3 minutes
- ultra-low sulfur of 15ppm or less
The Zeolitic Catalyst
Core of the process is the proprietary zeolitic catalyst.
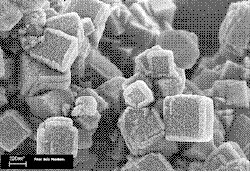 Zeolites (Greek, zein, "to boil"; lithos, "a stone") are minerals that have a micro-porous structure, forming some kind of a molecular grid. (Source: Wikipedia)
Zeolites (Greek, zein, "to boil"; lithos, "a stone") are minerals that have a micro-porous structure, forming some kind of a molecular grid. (Source: Wikipedia)
Zeolites are the alumino silicate members of the family of microporous solids known as "molecular sieves." The term molecular sieve refers to a particular property of these materials, for example the ability to selectively sort molecules based primarily on a size exclusion process. This is due to a very regular pore structure of molecular dimensions.
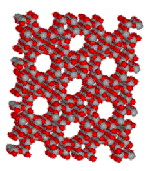 Synthetic Zeolites are widely used as catalysts in the chemical and petrochemical industry, for instance in Fluid Catalytic Cracking and Hydro-Cracking. Zeolites confine molecules in small spaces, which causes changes in their structure and reactivity. The hydrogen form of zeolites (prepared by ion-exchange) are powerful solid-state acids, and can facilitate a host of acid-catalyzed reaction, such as isomerisation, alkylation, and cracking.
Synthetic Zeolites are widely used as catalysts in the chemical and petrochemical industry, for instance in Fluid Catalytic Cracking and Hydro-Cracking. Zeolites confine molecules in small spaces, which causes changes in their structure and reactivity. The hydrogen form of zeolites (prepared by ion-exchange) are powerful solid-state acids, and can facilitate a host of acid-catalyzed reaction, such as isomerisation, alkylation, and cracking.
These zeolites, proprietary to the HC³ process, are suspended ("dissolved") in a carrier oil with high viscosity that is only slightly deteriorating by the aggressive catalyst at the temperature our unit is operated under. The oil is constantly and automatically replenished from the high viscosity, non-evaporative parts of the feedstock's hydro carbons.
The catalyst is not so lenient to the lighter hydrocarbons of the feedstock. As soon as the feedstock, heated by the carrier oil to a temperature between 450 and 650°F is exposed to the catalyst, the long chains, weakened by the moderate heat, break up and form diesel fumes, which are fed to a distillation column, where they are converted to a fluid state and mixed to a light oil with all the properties of commercially available No. 2 diesel. Even better, the output fulfills all requirements of ULDS, the new ULTRA LOW SULFUR DIESEL of 15ppm sulfur contents and a Cetane number of 60+.
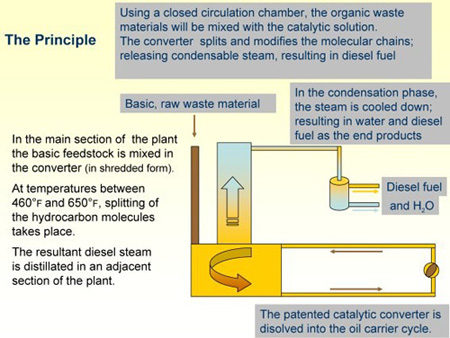
The Turbine
The patented turbine is the core of the system. The patent was granted in March 2006 and is valid throughout the world. It will protect this process for the foreseeable future and contains 19 claims, which were all undisputed. All other methods and experimentes in the world use:
- high temperatures
- and/or extremely high pressures
The result is not only a poor efficiency and an unfavorable energy balance = high costs, but the method of heat transfer leads to massive and solid carbon buildup, which soils the product and leads to production interruptions. This effect is caused by convection heat—a boiler employs a 'hot plate' (wall of a tank) to transfer the heat from a flame into the medium.
According to the 2nd law of thermodynamics the hot plate always has to have a higher temperature than the medium to be heated. Imagine a "pot of stew"—if it is not constantly stirred well, it will burn at the bottom. The same happens in chemical plants, for example an acetylene plant. The result is clogging by solid carbon to an extent that the facility has to be regularly stopped for maintenance, the so called "de-coking." It is a dangerous process, due to fire hazard and results in a loss of productivity.
Pyrolisis and Fischer-Tropsch all have to fight with this problem, which leads to contamination of the finished product that has to be filtered afterwards.
The HC³ Turbine avoids these problems. The heat is produced by a slight friction between the turbine vanes, which are surrounded by the carrier oil for the feedstock.
This oil has two purposes—dispersion of the shredded feedstock and heat transfer from the vanes into the material.
As the temperature increase is slow and the combined surface of all vanes large, the small amount of heat is immediately absorbed by the carrier oil. Combined with the low process temperature of only 650�F, which is below the coking temperature, no solid carbon buildup occurs.
A highly positive energy balance and no soiling of the product are the result.
 Zeolites (Greek, zein, "to boil"; lithos, "a stone") are minerals that have a micro-porous structure, forming some kind of a molecular grid. (Source: Wikipedia)
Zeolites (Greek, zein, "to boil"; lithos, "a stone") are minerals that have a micro-porous structure, forming some kind of a molecular grid. (Source: Wikipedia) Synthetic Zeolites are widely used as catalysts in the chemical and petrochemical industry, for instance in Fluid Catalytic Cracking and Hydro-Cracking. Zeolites confine molecules in small spaces, which causes changes in their structure and reactivity. The hydrogen form of zeolites (prepared by ion-exchange) are powerful solid-state acids, and can facilitate a host of acid-catalyzed reaction, such as isomerisation, alkylation, and cracking.
Synthetic Zeolites are widely used as catalysts in the chemical and petrochemical industry, for instance in Fluid Catalytic Cracking and Hydro-Cracking. Zeolites confine molecules in small spaces, which causes changes in their structure and reactivity. The hydrogen form of zeolites (prepared by ion-exchange) are powerful solid-state acids, and can facilitate a host of acid-catalyzed reaction, such as isomerisation, alkylation, and cracking.